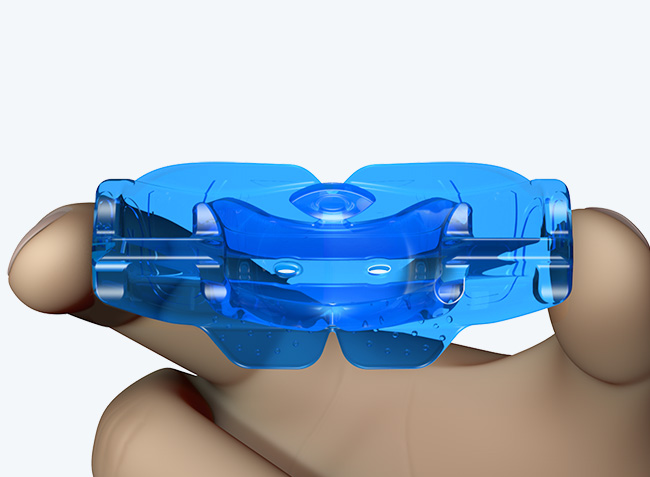
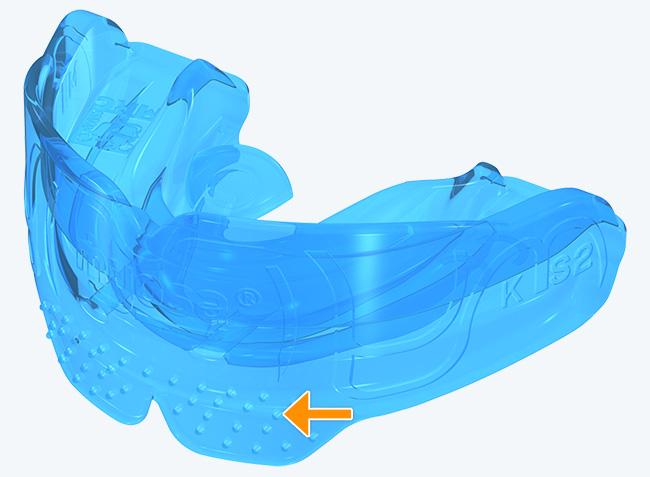
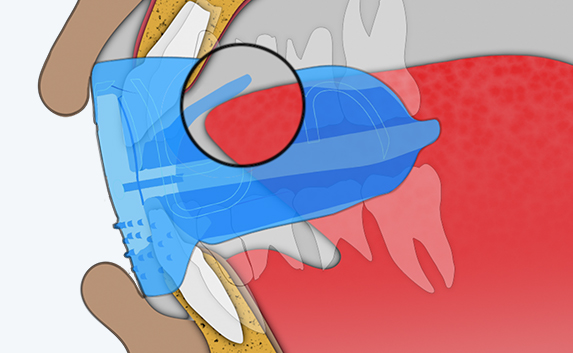
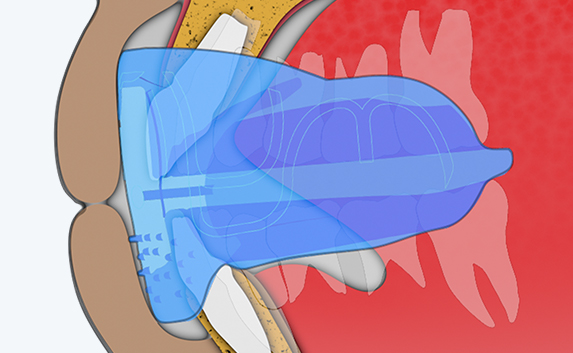
Myosa® for Kids Stage 2 - KS2
Establish nasal breathing and habit correction
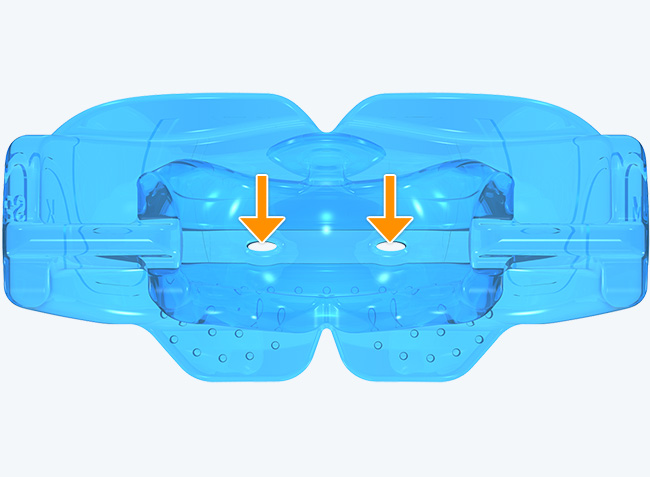
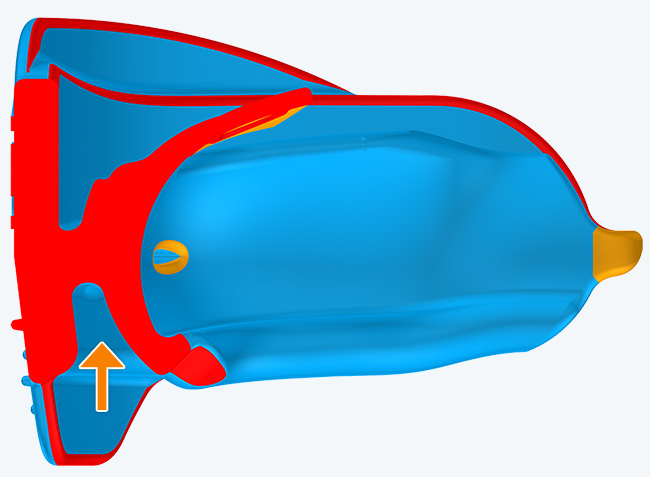
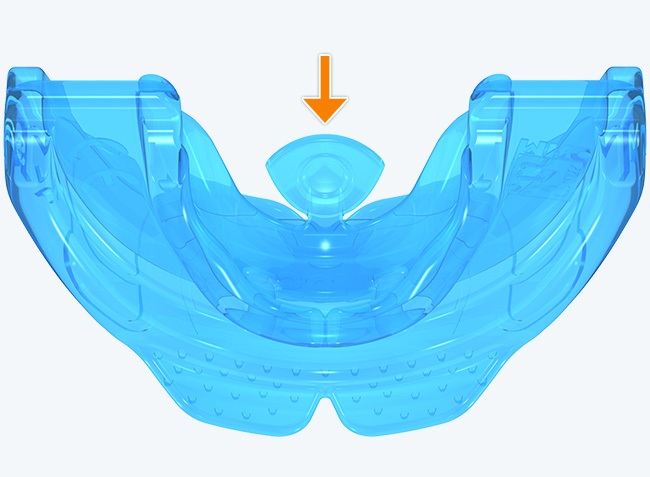
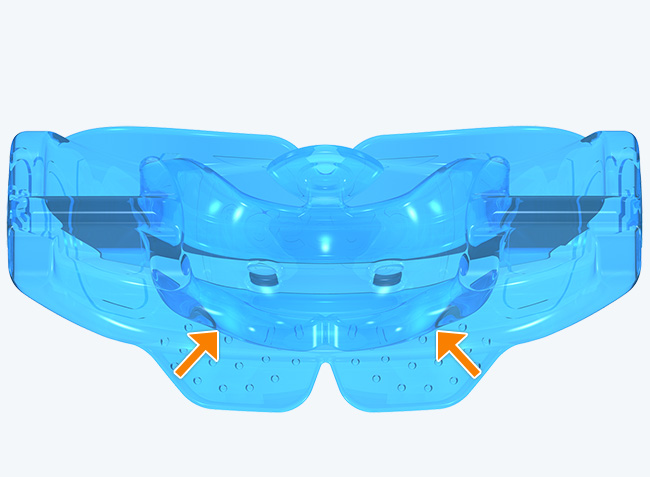
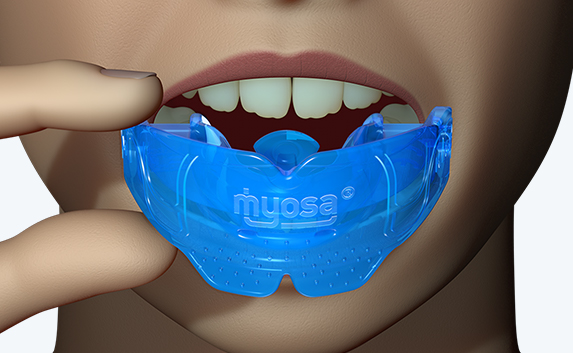
The KS2 focuses on establishing nasal breathing and correcting myofunctional disorders in children who are mouth breathers and have poor myofunctional habits. It is used in the mixed dentition stage and has small breathing holes to establish nasal breathing and myofunctional features to promote correct habits. Upon correction of breathing and myofunctional disorders, the patient may progress to The Myobrace® System to focus on myofunctional orthodontic treatment, designed to improve orthodontic problems for further habit correction.
Cleaning the Appliance
The Myosa® appliance should be cleaned under warm running water every time the patient removes it from their mouth.
Use Myoclean™ tablets to thoroughly clean twice a week. Myoclean™ is the recommended cleaning agent for all MRC appliances.

Resources
Appliance Instructions
Downloadable pdf document with instructions specifically for the KS2.
Download ResourceMyosa® Appliance Catalogue
Downloadable pdf document detailing the Myosa® appliance range.
Download ResourceContraindications, Warnings and Precautions
Contraindications
You should NOT use Myosa® if you have:
- An allergy or sensitivity to device materials.
- Tooth mobility or advanced periodontal disease.
- Uncontrolled dental disease.
- Multiple missing molar teeth.
- Complete nasal obstruction, unless by prescription from a healthcare provider.
- Central Sleep Apnea.
- Severe respiratory disorders.
Do not use with braces or dental aligners.
Warnings
Use of the Myosa® appliance may cause:
- Excessive salivation or dry mouth during initial treatment;
- Discomfort in the teeth and gums;
- Pain or soreness to the temporomandibular joint;
- Tooth movement or changes in dental occlusion;
- Obstruction of oral breathing.
Notify your dentist if you notice any signs of tooth movement, changes in your bite, persistent pain or discomfort, or if you had trouble fitting the appliance.
Precautions
THIS IS A PRESCRIPTION ONLY DEVICE AND MUST ONLY BE ISSUED BY A LICENSED PRACTITIONER.
This product is not intended to treat sleep apnea, as serious condition. Disclose any medical history of asthma, breathing, or respiratory disorders, or other relevant health problems to your medical practitioner before starting treatment.
Caution
Discontinue use immediately at the first sign of appliance damage or deterioration (including but not limited to splitting, tearing, contamination and discolouration)and consult your health practitioner. It is recommended to replace the appliance after 6 months of use.